view the rest of the comments
Lemmy Shitpost
Welcome to Lemmy Shitpost. Here you can shitpost to your hearts content.
Anything and everything goes. Memes, Jokes, Vents and Banter. Though we still have to comply with lemmy.world instance rules. So behave!
Rules:
1. Be Respectful
Refrain from using harmful language pertaining to a protected characteristic: e.g. race, gender, sexuality, disability or religion.
Refrain from being argumentative when responding or commenting to posts/replies. Personal attacks are not welcome here.
...
2. No Illegal Content
Content that violates the law. Any post/comment found to be in breach of common law will be removed and given to the authorities if required.
That means:
-No promoting violence/threats against any individuals
-No CSA content or Revenge Porn
-No sharing private/personal information (Doxxing)
...
3. No Spam
Posting the same post, no matter the intent is against the rules.
-If you have posted content, please refrain from re-posting said content within this community.
-Do not spam posts with intent to harass, annoy, bully, advertise, scam or harm this community.
-No posting Scams/Advertisements/Phishing Links/IP Grabbers
-No Bots, Bots will be banned from the community.
...
4. No Porn/Explicit
Content
-Do not post explicit content. Lemmy.World is not the instance for NSFW content.
-Do not post Gore or Shock Content.
...
5. No Enciting Harassment,
Brigading, Doxxing or Witch Hunts
-Do not Brigade other Communities
-No calls to action against other communities/users within Lemmy or outside of Lemmy.
-No Witch Hunts against users/communities.
-No content that harasses members within or outside of the community.
...
6. NSFW should be behind NSFW tags.
-Content that is NSFW should be behind NSFW tags.
-Content that might be distressing should be kept behind NSFW tags.
...
If you see content that is a breach of the rules, please flag and report the comment and a moderator will take action where they can.
Also check out:
Partnered Communities:
1.Memes
10.LinuxMemes (Linux themed memes)
Reach out to
All communities included on the sidebar are to be made in compliance with the instance rules. Striker

But sugar dissolves in cold water. It just takes a bit longer. This is 9th grade chemistry. At 20°C 203.9g sugar are soluble per 100ml of water.
[Edit: Sorry, for the Americans here: At 68°F, 1 cup of sugar is soluble in 21/50 cups of water.]
Wikipedia (de): Zucker cites Hans-Albert Kurzhals: Lexikon Lebensmitteltechnik. Volume 2: L – Z. Behr, Hamburg 2003, ISBN 3-86022-973-7, p. 723.
And most of all, solubility being a function of the temperature, if you lower it the excess sugar will leave the solution and cristallize.
I came here to say this, but the best Aqua is without sugar anyway.
Preach
It takes time for that to happen and in the meantime you can have a gross oversaturated solution.
Edit: not even oversaturated, would just take a long time for all that sugar to dissolve unless it's hot.
Have you seen how much sugar those hicks put into their tea though? It's gotta be hot because they put coca cola grade amounts of sugar, to the point where it wont dissolve in the water anymore. Sweet tea contains 36-38 grams of sugar per 16 oz. That's a fucking soft drink.
16 oz (454ml) can dissolve some 900 grams of sugar, far in excess of 38 grams. Sugar is ridiculously soluble in water.
Please attempt this and post results
It's easy. It's just making simple syrup.
The consistency alone is enough to know that sweet tea is nowhere near saturated.
Grams per ounce? You guys are savages with your units for concentration.
You just need to do more drugs
When I make my sweet tea, I use two cups per gallon, which comes out to about 50g of sugar per 16oz. And it’s delicious! It’s definitely not a “drink all the time” type drink. I only make it a few times a year for friends.
example: you don't make a pitcher of kool-aid with hot water.
however, adding sugar to the hot tea does work better than adding it after it's already chilled.
How? Wouldn't the excess sugar just come out of solution when the tea cools down again?
It dissolves quickly when the solution is warm. You would need to add a ridiculous amount for it to be saturated at room temp or slightly below.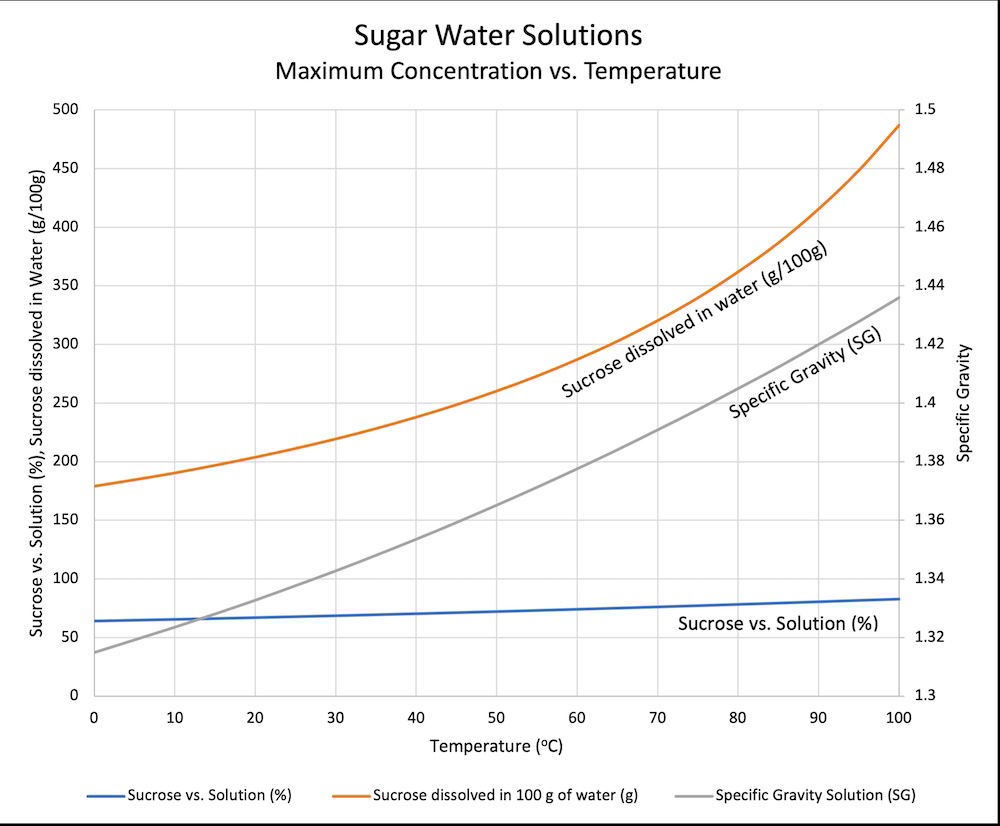
"ice cold" water can hold about 170 grams of sugar in 100 grams of water
If sweet tea drinkers could read they'd be very upset by that graph.
...is what I was going to say, but man it took me a while to figure out and I'm still not 100% sure I really understand it. The specific gravity line and the sucrose vs solution line are tied to the sucrose dissolved in water curve, right? Wait, the left axis is merging two different scales? Sometimes data really isn't beautiful.
The labels on the vertical axes match the labels on the lines. So the right vertical axis is for specific gravity (the grey line), and the left axis for the other two lines.
Ignore everything but the orange line and the left y-axis. It's just showing the weight of sugar that fits in 100g of water, vs temperature. The blue one shows that value as a percentage, g sugar divided by total sugar and water.
Right but you're forgetting there are already other things dissolved in the water as their not using pure, de-ionized water, and they're adding in tea.
I don’t think the ions and “tea molecules” really matter compared to 170g of sugar. Does a glass of water get notably heavier after adding in tea?
correct
Tap water usually sits around 200 ppm or 0.02% minerals. The tea leaves themselves, as I make my tea, are around 10g/L. Say the leaves dissolve 10% as an overestimation. That gives you water with 0.1% tea, 0.02% other. The solubility limit for sugar is 63% (by mass).
In general, the amount of salts or other organic molecules do not affect the solubility of sugar (or any other solute). The solubility of any solute in water is a constant (for a given temperature), as long as whatever is already dissolved does not have any compounds or ions in common with the next solute.
For example, if we wanted to dissolve sodium chloride into a solution of potassium chloride, the amount of chloride already dissolved would affect the amount of NaCl we could dissolve. But if we wanted to dissolve NaCl into a solution of potassium iodide, the KI would have zero effect on the NaCl solubility.
So, since tea has zero molecules in common with the sucrose, the yes shouldn't affect the solubility of sucrose at all. The only exception would be if solution is acidic, the sucrose can break down into glucose and fructose, of which the tea may have a small (negligible) amount.
Plus we're not actually saturating the sweet tea. Saturated sugar water is a syrup, so you know just by the consistency that sweet tea is nowhere near saturated.
Good details. Thanks Niels Bohron lol
They're not super saturating it. They're putting an amount of sugar in the tea that can dissolve at room temperature, it just takes a long time to do so.
Ok, got it. Someone in this thread mentioned ice cold water can still hold 1.7x its weight in sugar.
Yeah basically, leave the pitcher to evaporate and you get your sugar back as a coating on thr glass
That's very thoughtful of you to provide the imperial measurements as well for Americans ☺️