I wanted to tell a joke here, but all the good jokes argon.
That was such a noble thing to admit.
it didnt get a reaction out of me
You could say you were inert to my attempt at humor.
The original joke was pure Gold, but these later ones are just Boron me
K
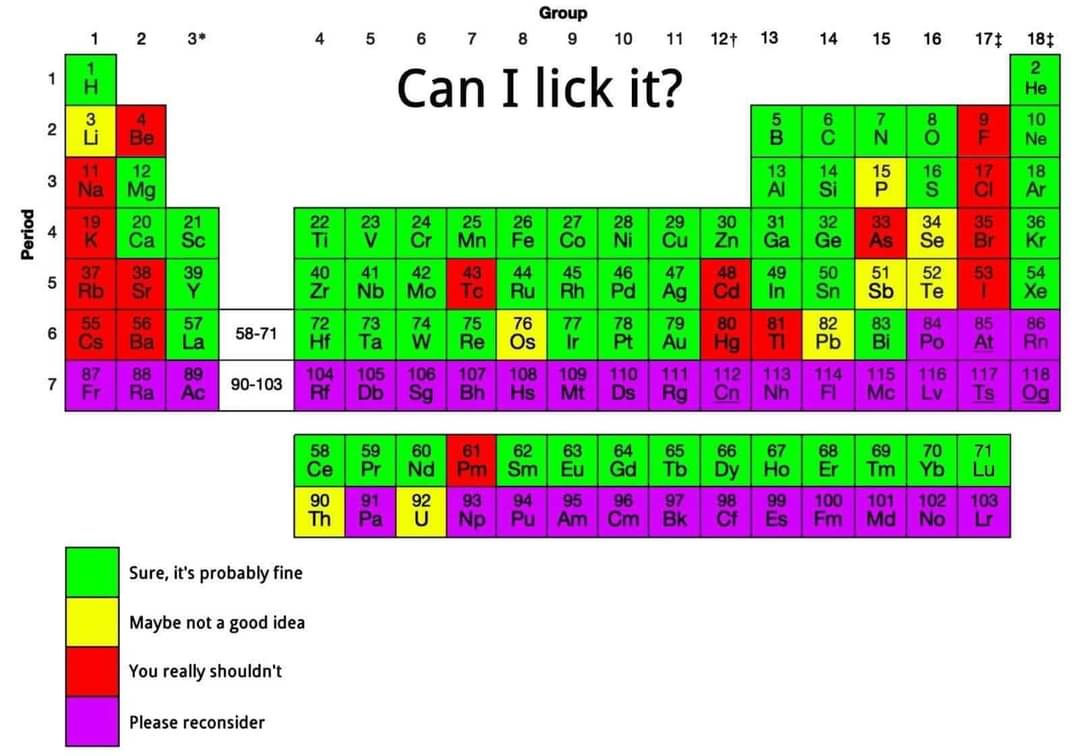
We can't lick sodium or chlorine, but combine them and you get something we literally make blocks of for the purpose of licking. What a world!
One is bad in one way and the other is bad in the opposite way.
Neutralize!
Remind your cousin Becky about this when she starts going on about mercury compounds in vaccines
This is like the nile red videos where hes like "plastic gloves are essentially grape fruit" and then proceeds to make it.
But does this imply licking it in a "lickable" state? I have a hard time imagining licking a gas, and licking hydrogen as a liquid at -250 C or so sounds, not great.
Depending on the quantity and the leidenfrost effect, you might be fine
That's hilarious because me and my brother licked lead fishing weights for fun as a child. It's probably why I'm retarded.
Can someone make one for suitability as dildo material?
Edit: Here it is, chumps

How is bromine "probably fine"? It should be in the rectal damage section.
Calcium should probably be in the "Ow, my ass" section.
There are a bunch wrong. Feel free to go crazy with it.
Edit: NEW VERSION IS UP Yay
awesome contribution
Lithium, Sodium etc. need to be upped to "please reconsider." Calcium and all the lanthanides are also metals I would not advise licking because theyre very reactive. Promethium is especially dangerous due to its radioactivity with its longest lived isotope having a half life of around 17 years. So not only is it reactive, youd die to the radiation too.
Lithium is just gonna be a little fizzy like pop rocks. No explosions, thankfully. The LiOH produced would not be fun for you, but probably won’t hurt anyone else.
Lithium salts are used to treat bipolar. The metal isnt just reacting with the water on your tongue to create a very strong base (and lots of heat), you are also going to be ingesting that Lithium (as a lithium soap as it reacts with oils and fats) which can have different (unpleasant) effects on you depending on how much was ingested. If your kidney function is impaired, it gets worse.
Please don't lick elemental hydrogen.
Out of curiosity, what would happen if you do?
In the hypothetical, if one were able to lick elemental hydrogen in its atomic, rather than molecular form, it would have a few potential effects. The one that would concern me most would be its aggressive reactivity, ripping hydrogens away from anything that it could in order to achieve stability. This would potentially cause tissue damage both from the deprotonation and shift in pH.
Nothing, because you can have only one atom of it. Multiple will just form molecular hydrogen H2. That one hydrogen atom will aggressively rip of another hydrogen of a molecule of water for example, but it won't be noticeable.
I'd bump up cesium, rubidium, and probably potassium to "please reconsider", as I would not want to stand near you
A decent chunk of these are "how would you even?" and a few others are "you're doing it right now."
Licks calcium one time 
Lick my As! You chemists can't stop me from slobbering on every element.
Yes you can!
Instructions unclear for isotopes
What if I want to lick U-235?
But Lead tastes so good!
I wonder what metallic Sodium tastes like...
It tastes like hot hydrogen gas (that will quickly mix with oxygen and taste like superheated steam).
If that doesn’t get ya, it would taste like sodium hydroxide, and also soap. (The soap is from the hydroxide turning the fats in your cells into soap.)
It tastes like pain.
Can I lick it?
Green - yes, you can!
Yellow, Red, Purple - no, you can’t!
I think licking pure uranium is worse for your health than licking pure chlorine gas
I think the assumption with the chlorine is that you end up inhaling it and dying fairly quickly. Licking uranium isn't a great idea, but you might not ever have noticable effects, even long term, if very little comes off onto your tongue. I know people who have accidently tasted plutonium in solution.
I dunno, if that gasses are in a state where they're able to be licked, they'd mess you up pretty bad
I'd say downgrade Mercury to yellow. Licking Mercury won't hurt you as long as you hold your breath.
Having it close to your breathy parts is always not a great idea though.
I would avoid licking zinc. It's a necessary nutrient but it doesn't take much to mess your stomach up.
My lead sandwich is calling to me
Mmm Pb&J.
I always wanted to play with bromine. It looks so cool.
Why all the coolest things have to be toxic 😞 (broad life wisdom statement)
Science Memes
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz