144
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
this post was submitted on 01 Sep 2024
144 points (98.6% liked)
chapotraphouse
13843 readers
620 users here now
Banned? DM Wmill to appeal.
No anti-nautilism posts. See: Eco-fascism Primer
Slop posts go in c/slop. Don't post low-hanging fruit here.
founded 4 years ago
MODERATORS
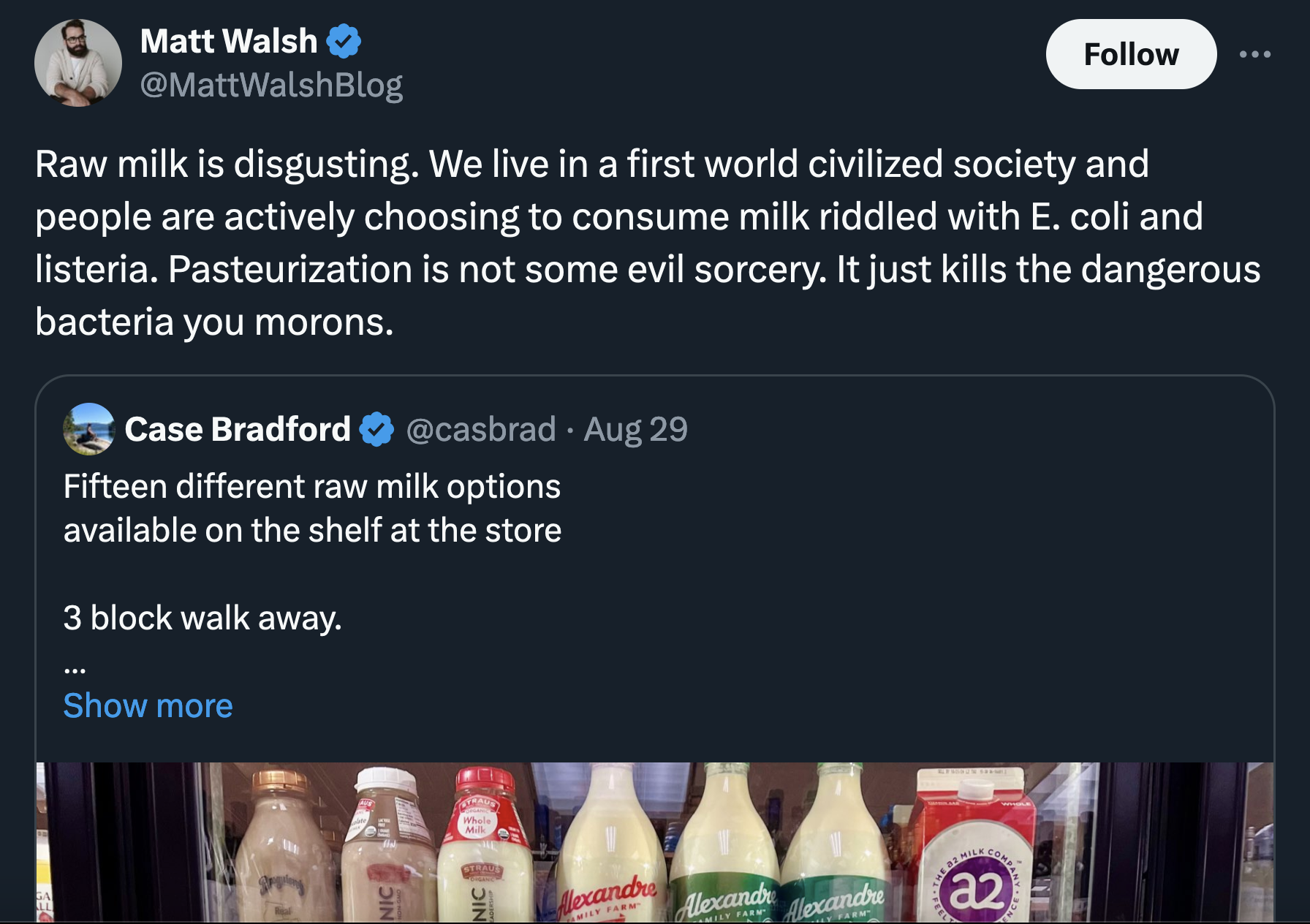
Most common antacids have a pH around 10, and for water to be alkaline it needs to have a pH between 8 and 9, so I guess you'd need 10 to 20 times more alkaline water than antacids for the same effect, as the ph scale increases at a tenfold level.
Would that even work? Intuitively (I dont remember anything about chem) I would guess that you could take a portion of a tums to get the same effect as alkaline water, but wouldn't be able to achieve the opposite. If you drank an enormous amount of alkaline water such that your stomach acid volume is negligible then the ph of the solution would just be the alkaline water ph, right?
Yeah you're probably right. It's just another reason this whole alkaline water thing makes no sense, on top of the fact that your body strictly regulates the pH levels of your blood.